Abstract
Background: The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies. INCB052793, a small molecule JAK1 inhibitor, is being evaluated in an ongoing phase 1/2 study of INCB052793 as monotherapy or in combination with standard therapies in patients with advanced hematologic malignancies. Preliminary safety and efficacy data are reported.
Methods: Phase 1 consisted of a monotherapy dose escalation (phase 1a) and combination therapy dose expansion (phase 1b). In phase 1a, patients with advanced hematologic malignancies received INCB052793 monotherapy (25, 35, and 50 mg QD). Phase 1b evaluated INCB052793 (25 and 35 mg QD) in patients with advanced multiple myeloma (MM) in combination with dexamethasone (DEX); or in patients with advanced acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or MDS/myeloproliferative neoplasm (MPN) overlap syndromes in combination with azacitidine (AZA). The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous 21-day (monotherapy) or 28-day (combination therapy) cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity. Phase 2 is evaluating INCB052793 combination therapy in patients with AML and high-risk MDS who failed prior therapy with hypomethylating agents (HMAs). Primary study objectives included safety, tolerability, and dose selection for expansion of INCB052793 monotherapy and combination therapy (phase 1) and safety and efficacy of INCB052793 combination therapy in patients with AML and higher-risk MDS (phase 2). Responses were recorded according to malignancy-specific criteria.
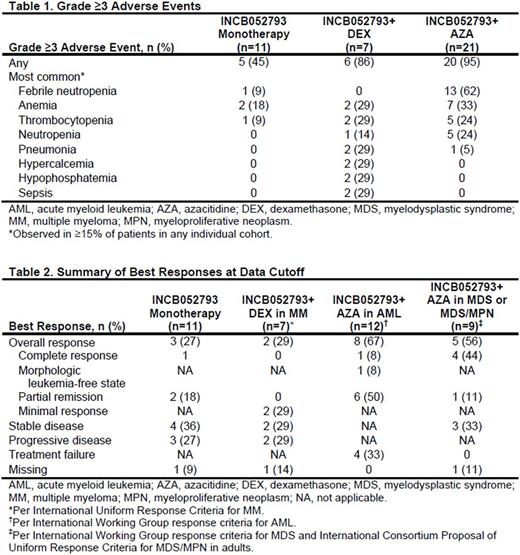
Results: We report data on the first 39 patients with hematological malignancies enrolled in the study.At data cutoff (June 19, 2017),11 patients (MDS/MPN, n=4; MM, n=3; diffuse large B-cell lymphoma, n=2; chronic lymphocytic leukemia, n=1; Hodgkin's lymphoma, n=1) received INCB052793 monotherapy. INCB052793+DEX combination therapy was received by 7 patients with MM; 21 patients received INCB052793+AZA combination therapy (AML, n=12; MDS, n=7; MDS/MPN, n=2). Prior HMA treatment was received by 0 patients in the INCB052793+DEX group and 71.4% (15/21) of patients in the INCB052793+AZA group. The median (range) duration of treatment was as follows: INCB052793 monotherapy, 104 (14‒528) days; INCB052793+DEX, 51 (15‒96) days; INCB052793+AZA, 125 (15‒456) days. Grade ≥3 adverse events (Table 1) were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793+DEX (most common: anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia), and 95% of patients receiving INCB052793+AZA (most common: febrile neutropenia, anemia, neutropenia, and thrombocytopenia). Most patients discontinued treatment (INCB052793 monotherapy, 91%; INCB052793+DEX, 100%; INCB052793+AZA, 90%), with the primary reasons being disease progression (INCB052793 monotherapy, 55%; INCB052793+DEX, 57%) or adverse event (INCB052793+AZA, 24%). Of 11 patients who received INCB052793 monotherapy, 1 with MDS/MPN had complete response (CR) and remains on study at data cutoff; 2 with MDS/MPN had partial remission (PR; Table 2). Of 7 patients with MM in the INCB052793+DEX group, 2 had a minimal response with a reduction in M protein. The overall response rate (ORR) was 67% (8/12) for patients with AML treated with INCB052793+AZA, with 1 CR, 1 morphologic leukemia-free state, and 6 PRs. The ORR was 56% (5/9) for patients with MDS or MDS/MPN who received INCB052793+AZA. Of 7 patients with MDS in the INCB052793+AZA group, 3 had CR. Of 2 patients with MDS/MPN in the INCB052793+AZA group, 1 had CR and 1 had PR.
Conclusion: Preliminary findings from this phase 1/2 trial indicate that INCB052793 has encouraging clinical activity, especially in combination with AZA, in patients with advanced myeloid malignancies, including those who previously failed HMAs. These data indicate that INCB052793 might (re)-sensitize HMA-refractory or relapsed patients to the effects of HMAs. Preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrolment is ongoing in phase 2 and expanded data, including PK/PD, will be presented.
Zeidan: Otsuka: Consultancy; Takeda: Speakers Bureau; AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria. Cook: Syros Corporation: Membership on an entity's Board of Directors or advisory committees. Bordoni: Merck: Honoraria, Speakers Bureau; Genentech: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Asatiani: Incyte Corporation: Employment, Equity Ownership. Zhou: Incyte Corporation: Employment, Equity Ownership. Faivre: Incyte Corporation: Employment, Equity Ownership. Byrne: Karyopharm: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Concert Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Savona: Sunesis: Research Funding; Incyte Corporation: Consultancy, Research Funding; Takeda: Research Funding; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Equity Ownership; Gilead: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal